Isaac’s just gone in for some routine surgery – removal of a dental cyst and a full eye exam under general anesthetic (his eyes have clouded over so badly that they can’t see in anymore, hence the more advanced look at things today.) For most families, that is routine. But for families dealing with MPS, anything under a general anesthetic is anything but routine, which is why Ellen, Gabriel, and I are sitting here, stressed and worried for our little boy.
Children suffering from MPS have compromised airways, and general anesthetics should be avoided at all costs. I’ve heard of too many complications that our beautiful kids have had while under a general to sit here and be relaxed about the process. MPS Specialists always recommend ensuring the best anesthesiologists handle our kids, and we are lucky to have the best today.
We are in the Surgical Waiting room at the Hospital for Sick Children, a place that I’ve grown accustomed to hating. The tension in this room is unbearable, and the waiting is worse. I can’t count the number of blog entries I’ve written from here – it gives me something to do to keep my mind off things.
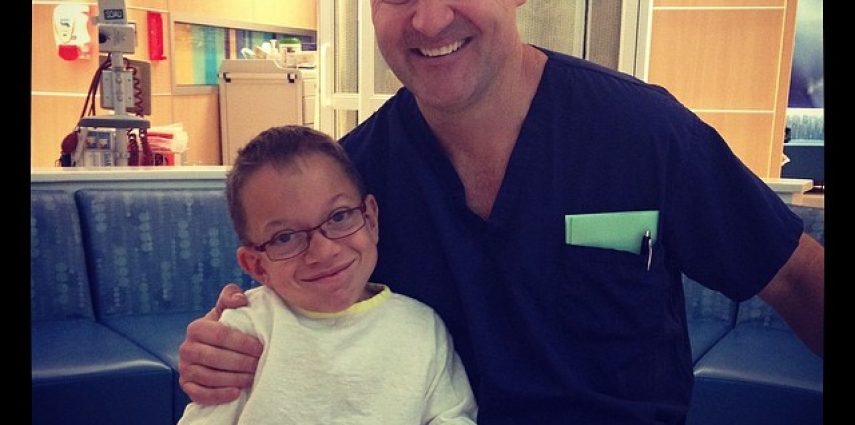
Parents and families dealing with MPS gain a unique perspective on life throughout the entire journey, and perhaps more so while sitting in a room like this. Sitting here, we’ve given up our child to the hands and arms of some of the best physicians in the world, and we have to trust that things will go smoothly. I’m comforted in the fact that Dr. Cengiz Karsli (pictured above with Isaac), an incredible anesthesiologist that has handled Isaac’s care since our first surgery here, is once again handling things for Isaac today. We were initially told that he wasn’t scheduled for Isaac today, and our stress level went through the roof. But he arrived and immediately made us feel better that he was there.
When Isaac was 2 1/2 years-old, he had a very major spinal-cord decompression surgery. The compression was so bad that they had to route out a piece of his vertebrae with a diamond drill bit. Needless to say, it was a very difficult surgery and we were terrified for our son. A few hours into the surgery, Dr. Karsli came into the waiting room looking calm and relaxed – he was actually chewing away on an apple and had a smile on his face. He dropped in quickly to tell us that things were going OK and not to worry. That moment made us admire him immediately. It was something he didn’t have to do, but he did so to put our minds at ease, and I’ve always been grateful to him for it. He probably doesn’t even remember that moment, but we sure do!
His relaxed nature is so helpful, but the kindness and care he shows our son really sets him apart from the rest. Even if Isaac’s airway doesn’t give him any trouble this morning, we’ll always do whatever we can to ensure that Dr. Karsli keeps Isaac under his care for the next surgery (and there will be more). If nothing else, this process is easier on us all with him being here, and we wouldn’t have it any other way.
Thinking back to our first surgery, this room felt so lonely for us. Ellen and I sat here worried sick for our son, and it felt like it was just us dealing with things on our own. We had just started our charity, and were trying to figure out how we were going to find a cure for our boy before it was too late. Today, 8 short years later, it feels like we have an army of support behind us, and this room doesn’t feel as lonely as it did before (I still hate it, however!) I posted a quick photo of Isaac earlier, and we’ve received so many words of hope and encouragement, and I’m incredibly thankful for that. And with that same help and support, we’re well on our way to finding a cure for our kids, and we can’ thank you all enough for being here for us always..
I’ll update once Isaac comes out of recovery and once I find a spare moment.
With Love and thanks always,
A.











 Michelle McCarthy for QMI Agency
Michelle McCarthy for QMI Agency
