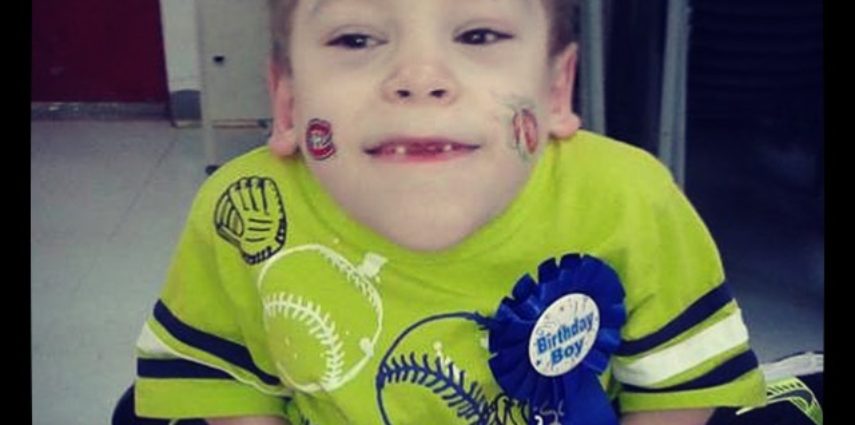
A young Baie Sainte Anne boy’s plight made it to the floor of the provincial legislature this morning.
Morgan Doucet, 10, is currently the only person in New Brunswick suffering from morquio syndrome.
On Wednesday morning, Southwest Miramichi-Bay du Vin MLA Jake Stewart called on the province reverse its decision not to fund a Health Canada approved treatment for Doucet’s illness.
According to the U.S. National Library of Medicine, morquio syndrome is an inherited disease of metabolism in which the body is missing or doesn’t have enough of a substance needed to break down long chains of sugar molecules called glycosaminoglycans (formerly called mucopolysaccharides).
Symptoms include:
– Abnormal development of bones, including the spine
– Bell-shaped chest with ribs flared out at the bottom
– Coarse facial features
– Hypermobile joints
– Knock-knees
– Large head (macrocephaly)
-Short stature with a particularly short trunk
– Widely spaced teeth
While Stewart didn’t name the treatment in question, the only Health Canada approved drug for the disease is Vimizim, which was approved last year.
Annual treatment can cost upwards of $100,000.
Stewart said Doucet needs treatment.
“Recently access to this lifesaving medication for Morgan Doucet was denied by the minister of health and the department, even though it is the gold standard of care as recommended by the international treatment guidelines, has been recommended by the Canadian Expert Panel on Morquio Syndrome and has been prescribed by Morgan’s genetic specialist at the IWK ” Stewart said in the legislature.
He accused the government of overruling the experts and said there is no process for appeal.
Health Minister Victor Boudreau said he could not discuss the specifics of the case on the floor of the legislature, citing privacy rules, but said their is a process in place for situations like Doucet’s.
“Because we do not have the level of expertise on these rare diseases and rare drugs here in the province of New Brunswick we rely on the province of Ontario and this is a process that was put in place by the member for Rothesay, the former minister,” Boudreau said.
He explained that the file is sent to experts in Ontario and they get back to the New Brunswick government with a recommendation.
But Stewart wasn’t happy with that reply.
“The drug that Morgan requires is currently being funded by Saskatchewan, Ontario and Quebec and in most developed countries. Morgan’s application was denied by a single reviewer from Ontario, a reviewer who no longer practices medicine and has never used this treatment,” Stewart said.
He also said Saskatchewan found flaws with the review process and with that reviewer. He said that province now deals directly with the experts on specific diseases.
“I ask the minister of health and the premier, will they show the same leadership and do the right thing by ordering a new review of Morgan’s file and provide immediate access to this life saving treatment while that review is taking place?” Stewart asked.
Boudreau said these are very tough situations.
“Everybody in this legislature has a heart and understands that these decisions are difficult to make,” Boudreau said.
“I can tell you that across the country, and in New Brunswick, some cases get approved, some cases don’t. We follow a process, Mr. Speaker. I am not an expert, and we don’t even have those experts within our department or within our province,” he added, saying the process was followed.